Inspire 2.0
Nyxoah and LivaNova are poised to compete in the US this year for OSA patients
You’ve probably seen the marketing for Inspire: late-night tv ads promising “no mask, no hose, just sleep.” These commercials have driven tons of patients to their sleep providers seeking out a seemingly minimally invasive option to treat their OSA.
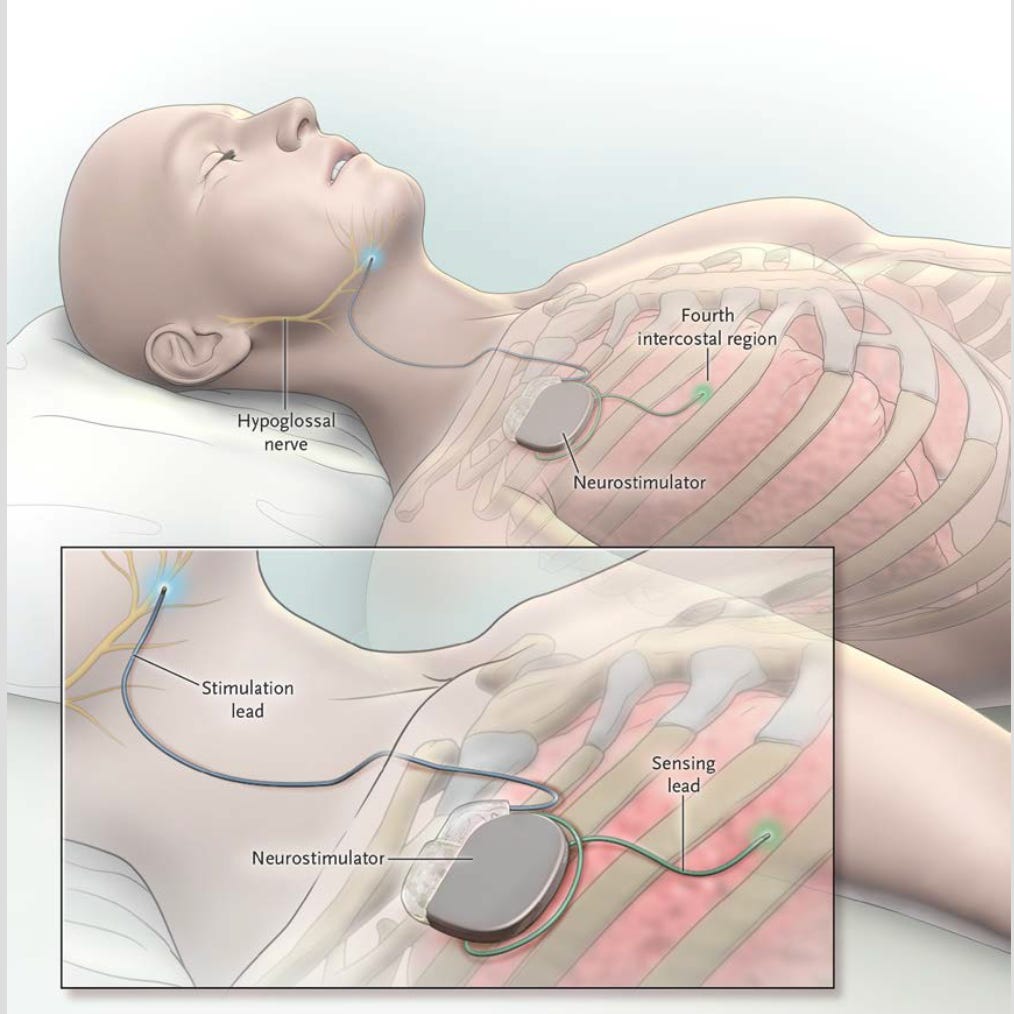
Since its FDA approval in 2014, the Inspire system has been the benchmark hypoglossal nerve stimulation (HNS) surgery, providing a worthwhile option for well selected patients intolerant to CPAP. However, two promising contenders—Nyxoah and LivaNova— are poised to compete in the 2.0 generation.
Inspire's Path
Inspire's HNS system operates by delivering electrical stimulation to the hypoglossal nerve controlling tongue motion - by synceing with respiration, the tongue stiffens during inspiration to maintain airway patency during sleep. First mover strategy and aggressive marketing have fueled growth for the past 10+ years and it has demonstrated overall safe and effective apnea-hypopnea index (AHI) reduction in well-selected CPAP-intolerant patients with moderate-to-severe OSA. It is worth stating reported outcomes still need to be better standardized. To date, over 100,000 patients have been implanted, underscoring its acceptance and effectiveness.
Despite this success, Inspire's system requires specific and careful preoperative screening, as well as a well-structured long-term follow-up strategy and fine-tuning beyond the initial device activation to optimize outcomes. Certain patients can’t tolerate the stimulation or have inadequate improvement, and there are device related issues. There is appetite for additional surgical options for/by patients. That is where Nyxoah and LivaNova fit in.
Nyxoah's Genio System
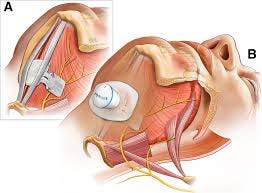
Design: The Genio system is a battery-free, leadless device implanted via a single submental incision. This differentiates it from Inspire. By delivering bilateral stimulation, the device is felt to have some unique advantages in efficacy, especially amongst patients with complete concentric collapse (CCC), who are currently NOT Inspire candidates.
Clinical Data - DREAM Study: initial data presented in 2024 demonstrated that the Genio system met its primary endpoints, showing statistically significant reductions in AHI and oxygen desaturation index (ODI) at 12 months. Notably, there was a 70% reduction in supine AHI, indicating efficacy regardless of sleep position.
This differentiated design and data in CCC patients is certainly intriguing. We have heard that there is a significant learning curve for the device placement and there are post-operative issues to consider (the DREAM study had a somewhat high adverse event rate, in their small overall N). We look forward to learning more.
LivaNova Aura6000
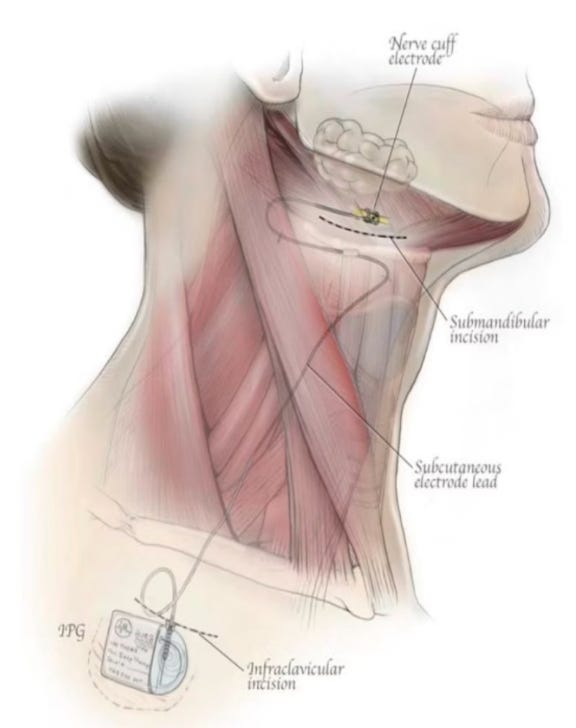
Design: Similar to Inspire, this device works on the hypoglossal nerve unilaterally. It has differentiated technology that is placed proximally (easier dissection/placement) and six circumferential electrodes that work to stiffen the tongue and prevent collapse. The battery needs to be charged by an external unit.
Clinical Data - OSPREY Study: The OSPREY trial reported 12-month data on May 7, 2025. In short, similarly solid data to Nyxoah: 65% surgical success, 68% reductions in AHI/ODI, clinically meaningful improvements in quality of life measures, no serious adverse/device related events.
LivaNova's simplified technique and expanded eligibility (also includes CCC patients) make it another interesting treatment option.
Looking Ahead: A Promising Time for OSA Patients
The advent of Nyxoah's Genio and LivaNova's Aura6000 systems - and a number of other companies developing new form factors and neural targets - signifies the next step in surgical OSA treatment in the United States:
Diverse Options: Patients will soon have access to a range of HNS therapies tailored to their specific needs and anatomy.
Innovation Trajectory: The trend towards less invasive, more patient-friendly devices is starting, with future iterations (version 3.0) likely to focus on miniaturization, new anatomic targets (ex. ansa cervicalis) and less invasiveness.
Clinical Implications: These advancements could lead to increased adoption of HNS therapy to a larger set of OSA sufferers, improved patient adherence, and better overall outcomes in OSA.
Stay tuned for more updates on the evolving landscape of sleep apnea treatments. Disclosures: Robson Capasso is an advisor for Restera Medical