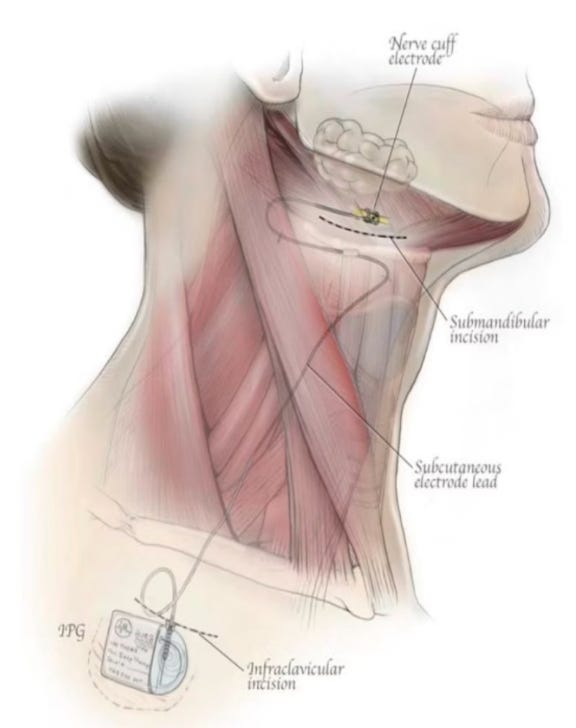
LivaNova Aura6000
What We Know and What Gaps Remain About a Third Hypoglossal Nerve Stimulator Device Option
A few months ago, LivaNova released 12-month top-line results from its OSPREY trial of the aura6000 system for moderate to severe obstructive sleep apnea (OSA). The study showed significant improvements in line with Inspire and Nyxoah devices, and positions LivaNova to be a 3rd FDA approved hypoglossal nerve stimulation device for OSA. This device works via “proximal” stimulation with multiple electrodes on the hypoglossal nerve: the company feels this may offer better control/tuning that could translate into fewer “non-responders” and more uniform performance. Here’s what we know so far about the device, what makes aura6000 interesting, and what gaps remain.
What the Data Says
Responders: 65% of patients in the treatment arm were “responders” at 12 months. That is, they achieved both at least a 50% reduction in their AHI (apnea-hypopnea index) and brought their AHI below 20.
AHI / ODI reductions:
Median AHI dropped by 68%, from 34.3 at baseline to 11.0.
Median oxygen desaturation index (ODI) fell by about the same magnitude (68%), from 34.9 to 11.1.
Patient-reported outcomes: Improvements in daytime sleepiness (Epworth Sleepiness Scale) and functional outcomes of sleep (FOSQ) were meaningful over the 12 months.
Safety: No serious device- or procedure-related adverse events reported during the trial.
What’s Particularly Promising
The OSPREY trial did not exclude complete concentric collapse (CCC) patients in their study population. The study utilized a predictive algorithm post-implants to estimate CCC patients in their study population, and found no significant difference in results:
“Response rates and AHI reductions at month 12 for patients in OSPREY with predicted risk for CCC were consistent with the results for the full study population, demonstrating the robustness of the therapeutic response.”
The company also highlights that OSPREY patients have a higher BMI and AHI severity than other HNS trials, giving them confidence on the applicability of the technology in a more broad OSA population.
“The OSPREY trial demonstrated rapid and sustained improvement for patients who received active proximal hypoglossal nerve stimulation, including those with severe obstructive sleep apnea, elevated body mass index, and high risk of complete concentric collapse,” said Ahmet Tezel, Ph.D., Chief Innovation Officer of LivaNova.
What We Need to Watch
Longer-term data beyond 12 months: Inspire has been FDA approved 10+ years, so we’ll want to ensure ongoing reliability, battery performance, and ongoing safety.
Real-world outcomes: in OSPREY, some patients showed benefit rapidly (≈25% response “day one”), ~50% by month 3, and continued improvement through the year. Similar to the other HNS devices, seems ongoing titration/maintenance is the rule not the exception.
Cost, reimbursement, and how payors will compare aura6000 vs existing options. In particular, will the device receive an indication for all moderate/severe OSA, without need for DISE. This would be a very impactful workflow change. Nyxoah is also pursuing this in their ACCCESS study.
Whether aura6000 can overcome physician familiarity and inertia (Inspire has established patient base, physician training, reputational lead).
LivaNova’s field safety notice says the implant “will need to be charged at least twice a week and possibly daily,” with each session typically ~30 minutes to 2.5 hours. The implant’s battery life can be up to ~15 years.
Analysts anticipate FDA approval in first half of 2026.
Summary / What This Means for the Landscape
Competition is heating up. With Genio now FDA-approved, Inspire no longer has the field to itself. Aura6000 has strong data to give it a chance to claim market share.
Potential for expanded access. If aura6000 is approved treat patients who were previously excluded (CCC), that shifts the denominator of people eligible for HNS therapy significantly, and definitely impacts patient workflows.
Patient choice. More implant types, more flexible options (levels of invasiveness, hardware footprint, external vs internal components) may allow more personalized decisions, e.g. what trade-offs a patient is willing to make in terms of battery, external gear, electrode configuration, etc.
It continues to be an exciting time for sleep clinicians and surgeons. We look forward to sharing more updates to the surgical/HNS landscape.
Best, Chris and Robson.