Surgeon's Guide: Inspire
Hypoglossal Nerve Stimulation (HNS)
Surgeon’s Guide: Inspire Hypoglossal Nerve Stimulation (HNS)
Over the past decade, Inspire has become the most well-known—and most requested—surgical option for patients with obstructive sleep apnea (OSA). For more information on the data around its use, and the next wave of hypoglossal nerve surgical options coming, please refer to our prior post: Inspire 2.0.
As a sleep surgeon, we’ve seen firsthand how much patient demand has grown for this therapy. Here’s a guide to the science, surgical details, and what patients should expect.
What Is Inspire?
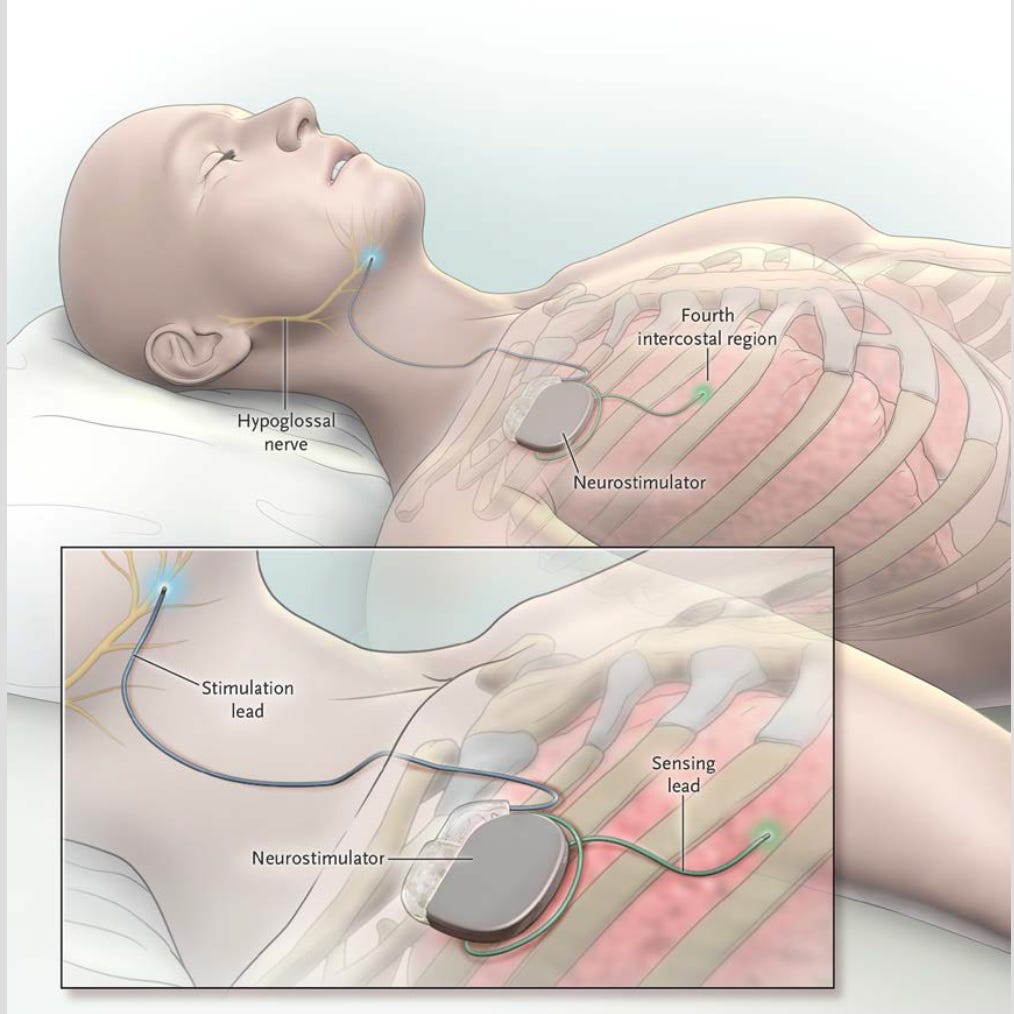
Inspire is an implantable medical device that treats OSA by stimulating the hypoglossal nerve, which controls tongue movement. This is synced to your respirations, so as you INSPIRE (when you’ll typically collapse the airway in sleep), the tongue pushes-forward and stiffens to maintain airway patency.
Think of it like a pacemaker for your tongue.
Patient Selection is Key
Inspire is meant for patients who:
Have moderate to severe OSA (best data AHI 15–65, though approved higher)
Are not significantly obese (best data is BMI <32, though FDA-approved up to BMI 40)
Have failed or cannot tolerate CPAP
Have favorable airway anatomy based on Drug-Induced Sleep Endoscopy (DISE)—specifically, no complete concentric collapse (CCC) at the palate
Separately, I (CG) have noticed that patients with insomnia often have difficulty tolerating the nerve stimulation during sleep. I have unfortunately had patients with great tongue motion who ‘don’t even feel it’ while awake that when sleeping describe activation waking them up. Because of this, I have treated that as a near contraindication to the surgery.
What the Procedure Involves
Inspire surgery is performed under general anesthesia and typically takes 1.5–2 hours. It involves two incisions, each about 5cm long (roughly the length of a AA battery):
Upper Chest (right side, under the collar bone): To place the pulse generator
Neck (under jaw): To place the stimulating lead on the hypoglossal nerve
These components are tunneled and connected under the skin. Most patients go home the same day. There is a battery powering the device that lasts ~10 years (at that time, an exchange procedure for the battery unit would be required).
What Is Recovery Like?
Discomfort: Mild to moderate, usually managed with over-the-counter pain medication
Downtime: Most patients return to non-strenuous activity within a few days; no heavy lifting for 2–3 weeks. I typically tell patients no arm stretching, repeated neck or body turning (yoga, truck drivers, golfers, etc.) for 1 month.
Device activation: Occurs ~1 month after surgery
Once activated, patients will home titrate the device for several weeks. For some patients, multiple additional device setting changes and fine-tuning is required. Eventually, we conduct a postoperative sleep study.
Does It Work?
Yes—very well, in the RIGHT patients. Data from clinical trials and real-world use show:
AHI reductions of 60–80%
Improvements in snoring, daytime sleepiness, and quality of life
Long-term durability
Overall good patient satisfaction
It’s not a cure, but an effective management tool.
What to Expect After Surgery
Pain
Typically mild and managed with acetaminophen for a week.
Diet
Typically a full general diet is tolerated. Sometimes a sore throat/tongue after the surgery makes patients prefer soft, cool, and soothing foods like smoothies, yogurt, and soups for the first week.
Activity
Doing normal activities (walking, preparing food, showering) is okay after surgery. We tell patients to minimize overhead arm movements and neck rotation during the first month post-op, to avoid device migration. Long-term, patients resume all normal activities.
Risks and Downsides
Like any surgery, Inspire carries risks:
Infection at incision/implant sites
Nerve injury (rare)
Need for battery replacement (every 10–11 years)
Device discomfort or stimulation side effects (often adjustable with reprogramming)
Cosmetic impact- 2 incision scars, possible visible cord in neck.
Less effective for breathing issues that happen while sleeping in supine/back position.
The biggest risk is that a patient can’t tolerate/use the device long-term or has unacceptable improvement in OSA. Again, patient selection is key.
🧐 Frequently Asked Questions About Inspire/HNS
1. Will Inspire cure my sleep apnea?
Not usually. The surgery is aimed at reducing the severity of OSA—not curing it. The goal is to improve symptoms, reduce health risks, and eliminate the need for CPAP in select patients.
2. How long is the recovery period?
Most patients can resume light activity in the first week. We recommend no heavy lifting, large arm/neck movements (golf, tennis, etc.) for greater than 1 month to allow device healing and stability.
3. What kind of anesthesia is used?
Inspire is performed under general anesthesia, meaning you’ll be fully asleep and monitored throughout the surgery. It is an outpatient surgery- you’ll go home the same day.
4. Can I travel with it?
Yes, including airport security. A medical ID card is provided.
5. Is it covered by insurance?
Yes, including Medicare and many commercial insurers. Prior authorization is typically required.
6. Is Inspire permanent?
Removal is possible but only if device complications/failures occur, or in some cases when MRI imaging of the chest or neck is required and a less potent 1.5T scanner is not an option. Battery replacement is required after ~10 years.
The Bottom Line
Inspire has emerged as a powerful option in the OSA treatment landscape. It isn’t for everyone—but for the right patient, it’s a life-improving solution when CPAP fails.
Stay tuned for more “Surgeon’s Guides” in this series—and if you’re new here, you can catch up on our other post: 👉 Surgeon’s Guide: Uvulopalatopharyngoplasty.
Until next time—sleep well.
—Chris and Robson.